Via 4Branch
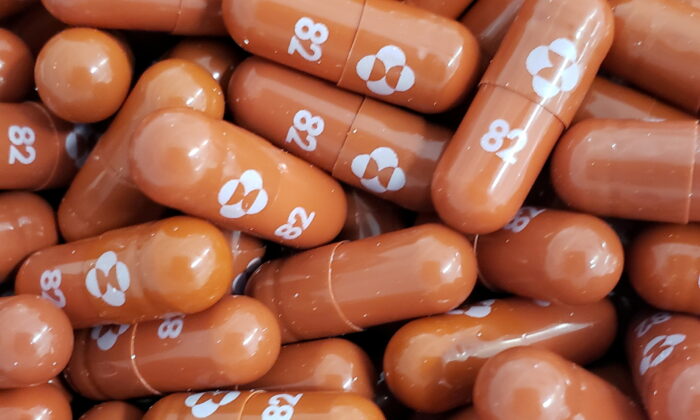
India’s top health research body announced on Wednesday that it won’t be adding Merck’s COVID-19 antiviral pill molnupiravir to its national treatment protocol, citing concerns over its safety.
The state-run Indian Council of Medical Research (ICMR) said it had become aware of “major safety concerns” that prompted the decision, despite India’s drug regulator in December approving the drug for emergency use.
More @ The Epoch Times

No comments:
Post a Comment